A Tauranga couple is splitting up their family so their daughter can access treatment for Spinal Muscular Atrophy (SMA) in Australia, which Pharmac won't fund in New Zealand.

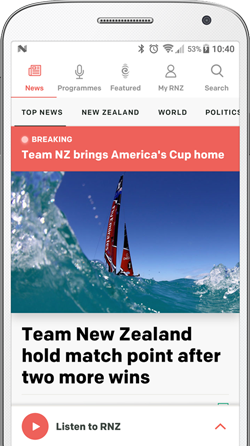
Maija Russell is moving to Australia with her mother, Jessica Russell, in order to get Spinraza for Spinal Muscular Atrophy, which Pharmac won't fund. Photo: RNZ/ Vinay Ranchhold / Supplied
Jessica Russell and her 16-year-old daughter Maija will move to Australia in October, while her partner stays in New Zealand to work, pay the mortgage and look after their teenage boy.
Maija is among about 35 New Zealand children with SMA, a deadly neurological condition which causes weakness and muscle wasting.
RNZ has learned that since 2018 about 10 families have left New Zealand to try to access SMA treatment overseas.
The Russells' move comes as the Human Rights Commissioner calls on Pharmac to urgently review funding for Spinraza, which can help treat SMA, saying the agency's assessment is too narrow and does not account for the true costs to society.
Australia, along with 56 other countries, provides public funding for Spinraza, which neurologists say has remarkable results combating SMA.
Pharmac has been considering an application to fund Spinraza since August 2018 and while its own expert advisory committee ranked it as a "high priority" in February 2020 Pharmac still will not pay for it.
Neurologists say time is vital with SMA because every day motor neurons are lost that cannot be regained.
"For Maija we are getting to a life and death situation," Jessica Russell says. "If we don't go now and get this treatment for her, the disease's progression for her has got to a really serious stage where she's losing the ability to swallow and it's impacting on her breathing."
Funding Spinraza privately would cost about $390,000 a year.
Chief Human Rights Commissioner Paul Hunt wrote to Pharmac in May calling for an urgent review of its refusal to fund the drug.
His six-page letter, also sent to the ministers of justice and health, was released to RNZ under the Official Information Act.
Hunt argued that Pharmac's approach was too narrow and did not take into account the costs to society - including housing, transport, education and employment - in allowing children like Maija to go untreated.
"I recommend Pharmac urgently evaluates SMA and Spinraza by way of a societal framework," Hunt wrote.
Russell says her family's experience backs up Hunt's approach.
Maijia was diagnosed in 2005 at six months old.
"They said, 'look, we think that this is Spinal Muscular Atrophy - don't Google it until we are sure'. And of course, you go home and Google it," Russell recalls.
"Our whole family's life just changed within half an hour."
For the first 10 years of Maija's life they visited Starship Children's Hospital about 10 times a year.
"A simple runny nose can within hours turn into life threatening pneumonia," she says.
"We were frequent fliers on the Westpac helicopter because things would literally go from a runny nose to severe respiratory distress within hours. So it was frightening."
In the three years that Pharmac has been considering whether to fund Spinraza, Maija's condition has deteriorated.
"During that time my daughter has lost all movement in her arms, she can no longer feed herself, scratch her nose and has lost the ability to eat solid food."
In October the family will split up so Maija can access the drug in Australia, spending much of their retirement savings on the move.

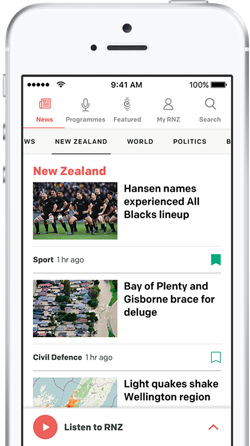
Fiona Tolich has dealt with 10 families who have moved overseas to access SMA drugs. Photo: RNZ / Cole Eastham-Farrelly
Fiona Tolich has SMA and has taken on an advocacy role with Patient Voice Aotearoa.
She has dealt with many of the 10 families who have left New Zealand to access Spinraza overseas.
"It's absolutely appalling," Tolich says.
"They feel that they are not worthy and they feel that they don't matter."
While Spinraza has a 'list price' of about $390,000 a year, drug industry experts say Pharmac can often secure a 50 percent discount through its confidential negotiating process. On that basis it would cost about $7 million a year to provide 35 children with Spinraza.

Paul Hunt Photo: University of Waikato
But Pharmac says because Spinraza's patent doesn't expire until 2030, it wouldn't be able to secure a 50 percent discount, so the cost would be "considerably higher" than $7 million, perhaps even three times as much.
Hunt accepts that "health rationing" is inevitable as Pharmac operates within a fixed annual budget of about $1 billion but he says it needs to broaden its funding criteria.
"Pharmac's evaluative model does not, for the most part, consider costs outside the health sector - it does not capture the cost to society of a medical condition."
Hunt's letter says other agencies do consider broader societal impacts under the government's 'wellbeing approach'.
In 2019 Finance Minister Grant Robertson delivered his first Wellbeing Budget, which demanded government departments work together, rather than in silos, and "move beyond narrow measures of success".
The Treasury has developed a Living Standards Framework to take into account wellbeing in its economic advice to the government.
"I see little evidence that Pharmac has adjusted its evaluation framework in light of the government's wellbeing approach and Treasury's Living Standards Framework," Hunt wrote. "In my view Pharmac's narrower approach is inconsistent with the government's wellbeing approach as well as the holistic approach to human rights."

Pharmac's Chief Exectuive Sarah Fitt says the corporation is unclear why it should give Spinraza special consideration Photo: RNZ / LUKE MCPAKE
Pharmac declined an interview with RNZ but did release the response it had sent to Hunt.
Pharmac argued it has not decided "not to fund Spinraza" it just has not funded it yet.
Spinraza, along with another SMA drug called Risdiplam, is on Pharmac's Options for Investment list of 102 drugs that its clinical advisors have recommended for funding - medicines it says it would like to fund if it had the money.
"We remain unclear what the justification would be for viewing Spinraza as a unique case worthy of special consideration," Pharmac chief executive Sarah Fitt says in response to Hunt.
Fitt says Pharmac's hands are tied by the law, which says it must focus on getting the best health outcomes.
"We consider there is limited ability within current legislation for us to consider wider societal factors beyond health."
Fitt told Hunt he should take his concerns to the panel conducting an independent review of Pharmac.
The review, announced by the government in March, will look at Pharmac's performance, decision making, transparency and progress towards equity, although the agency's budget is out of scope.
But Hunt says a fresh look at Spinraza is urgent and "should not and need not" wait for the Pharmac review.
Jessica Russell says her family cannot wait any longer and that is why she and Maija are now preparing for the move to Australia.
"We're not rich. It's a huge financial investment to get there. And we still have a mortgage to pay."
Maija does not travel light. They will need to take her breathing equipment, a hoist, a special chair she uses in the shower and a wheelchair lift - all before looking for a new house with wheelchair access.
"Even getting on a plane - from a wheelchair into the chair on the plane - is unbelievably complicated."
The risk of travelling during the Covid-19 pandemic adds another layer of complexity.
"It just blows my mind if I try and think of everything at once."
While the Human Rights Commissioner is pushing for Pharmac to consider the societal cost, Jessica Russell says there is an emotional cost too.
"You can't put a dollar figure on what this treatment would mean to someone with Spinal Muscular Atrophy," she says.
"It's a no brainer. It's the right thing to do. It's the moral thing to do. It's everything to us."